Product Details
| Species Reactivity |
General |
| UniProt |
N/A |
| Abbreviation |
PG-E2 |
| Alternative Names |
N/A |
| Range |
1-400 pg/mL |
| Sensitivity |
1 pg/mL |
| Sample Type |
Serum, Plasma, Other biological fluids |
| Detection Method |
Sandwich |
| Analysis Method |
Quantitive |
| Assay Duration |
1-4.5h |
| Sample Volume |
1-200 μL |
| Detection Wavelengt |
450 nm |
Test principle
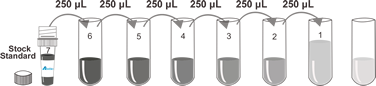
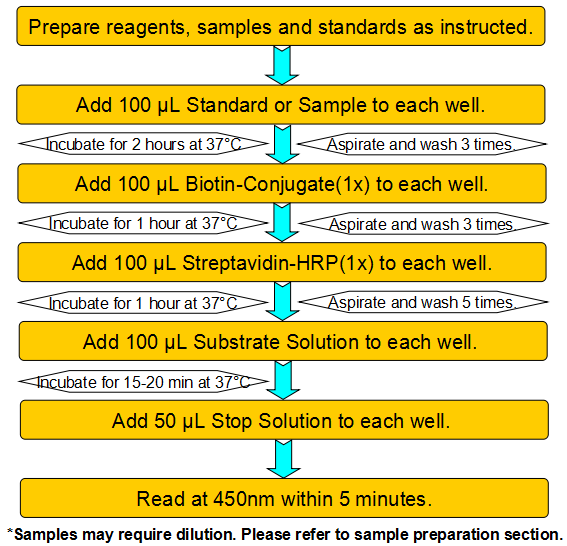
This assay employs a two-site sandwich ELISA to quantitate PG-E2 in samples. An antibody specific for PG-E2 has been pre-coated onto a microplate. Standards and samples are pipetted into the wells and any PG-E2 present is bound by the immobilized antibody. After removing any unbound substances, a biotin-conjugated antibody specific for PG-E2 is added to the wells. After washing, Streptavidin conjugated Horseradish Peroxidase (HRP) is added to the wells. Following a wash to remove any unbound avidin-enzyme reagent, a substrate solution is added to the wells and color develops in proportion to the amount of PG-E2 bound in the initial step. The color development is stopped and the intensity of the color is measured.
Product Overview
Prostaglandin E2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin which is used as a medication. As a medication it is used in labor induction, bleeding after delivery, termination of pregnancy, and in newborn babies to keep the ductus arteriosus open.In babies it is used in those with congenital heart defects until surgery can be carried out. It may be used within the vagina or by injection into a vein.Common side effects include vomiting, fever, diarrhea, and excessive uterine contraction. In babies there may be decreased breathing and low blood pressure. Care should be taken in people with asthma or glaucoma and it is not recommended in those who have had a prior C-section. Prostaglandin E2 is in the oxytocics family of medications. It works by binding and activating the prostaglandin E2 receptor which results in the opening and softening of the cervix and dilation of blood vessels.Prostaglandin E2 was first made in 1970 and approved for medical use in the United States in 1977. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. In the United Kingdom a dose costs the NHS about 8.50 to 30.00 pounds. In the United States a course of treatment costs more than 200 USD.Prostaglandin E2 works as well as prostaglandin E1 in babies; however, is much less expensive.
Components
Reagents |
Quantity |
Reagents |
Quantity |
Assay plate (96 Wells) |
1 |
Instruction manual |
1 |
Standard (lyophilized) |
2 |
Sample Diluent |
1 x 20 mL |
Biotin-Conjugate (concentrate 100 x) |
1 x 120 μL |
Biotin-Conjugate Diluent |
1 x 12 mL |
Streptavidin-HRP (concentrate 100 x) |
1 x 120 μL |
Streptavidin-HRP Diluent |
1 x 12 mL |
Wash Buffer (concentrate 25 x) |
1 x 20 mL |
Substrate Solution |
1 x 10 mL |
Stop Solution |
1 x 6 mL |
Adhesive Films |
4 |
Specificity
This assay has high sensitivity and excellent specificity for detection of Human PG-E2. No significant cross-reactivity or interference between Human PG-E2 and analogues was observed.
Recovery
Matrices listed below were spiked with certain level of recombinant Human PG-E2 and the recovery rates were calculated by comparing the measured value to the expected amount of Human PG-E2 in samples.
Precision
Intra-assay Precision (Precision within an assay)
Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays)
Three samples of known concentration were tested in forty separate assays to assess inter-assay precision.
CV (%) = SD/meanX100
Intra-Assay: CV<8%
Inter-Assay: CV<12%
Linearity
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of Human PG-E2 and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
Stability
The stability of ELISA kit is determined by the loss rate of activity. The loss rate of this kit is less than 5% within the expiration date under appropriate storage condition.
The loss rate was determined by accelerated thermal degradation test. Keep the kit at 37°C for 4 and 7 days, and compare O.D.values of the kit kept at 37°C with that of at recommended temperature. (referring from China Biological Products Standard, which was calculated by the Arrhenius equation. For ELISA kit, 4 days storage at 37°C can be considered as 6 months at 2 - 8°C, which means 7 days at 37°C equaling 12 months at 2 - 8°C).
Sample collection and storage
Serum: Use a serum separator tube (SST) and allow samples to clot for two hours at room temperature or overnight at 2 - 8°C before centrifugation for 15 minutes at 1000 × g. Remove serum and assay immediately or aliquot and store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Plasma: Collect plasma using EDTA, or heparin as an anticoagulant. Centrifuge for 15 minutes at 1000 × g at 2 - 8°C within 30 minutes of collection. Assay immediately or aliquot and store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Other biological fluids: Centrifuge samples for 20 minutes at 1000 × g. Remove particulates and assay immediately or store samples in aliquot at -20°C or -80°C. Avoid repeated freeze/thaw cycles.
Kits storage instructions
Store at 2-8°C. Please refer to Instruction Manual.