Current Position:Home>>Elisa Kit For Food Safety & Drug Residues>> Ciprofloxacin (CPFX) ELISA Kit
Cat.No.: AE81016SF
Welcome to order from local distributors.
Add to cart Bulk requestFor research use only. Order now, ship in 3-5 days
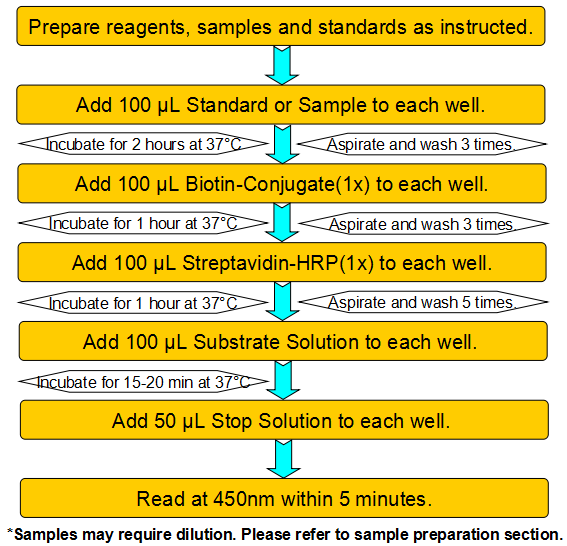

| Species Reactivity | N/A |
| UniProt | N/A |
| Abbreviation | CPFX |
| Alternative Names | N/A |
| Range | 0.1-8.1 ppb |
| Sensitivity | 0.1 ppb |
| Sample Type | Animal tissue, aquatic tissue, honey, etc. |
| Detection Method | Competitive ELISA |
| Analysis Method | Quantitive |
| Assay Duration | 1-3h |
| Sample Volume | �1�0�-�2�0�0� μ�L |
| Detection Wavelengt | 450 nm |
Reagents | Quantity | Reagents | Quantity |
Assay plate (96 Wells) | 1 | Instruction manual | 1 |
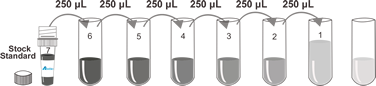
Standard | 6 x 1 mL | Redissolving Solution (concentrate 2 x) | 2 x 20 mL |
Antibody | 1 x 7 mL | HRP-Conjugate | 1 x 7 mL |
Wash Buffer (concentrate 20 x) | 2 x 20 mL | Stop Solution | 1 x 7 mL |
Substrate A | 1 x 7 mL | Substrate B | 1 x 7 mL |
2-Nitrobenzaldehyde (C7H5NO3) | 1 x 10 mL | Adhesive Films | 4 |
Sample Type |
Number |
Recovery range (%) |
Average(%) |
Serum |
10 |
90-101 |
96 |
EDTA plasma |
10 |
89-97 |
93 |
Heparin plasma |
10 |
91-99 |
95 |
Sample Type |
1:2 |
1:4 |
1:8 |
1:16 |
Serum |
78-89% |
81-99% |
92-103% |
95-105% |
EDTA plasma |
91-101% |
90-98% |
93-101% |
91-98% |
Heparin plasma |
92-103% |
93-102% |
92-99% |
91-101% |